When it comes to batteries, there are always areas for improvement: the race is on to develop batteries that are cheaper, safer, longer lasting, more energy dense, and easily recyclable.

Pathways Toward Realizing the Promise of All-Solid-State Batteries
In a review article published in the March 2020 issue of Nature Nanotechnology, nanoengineers at the University of California San Diego offer a research roadmap that includes four challenges that need to be addressed in order to advance a promising class of batteries—all-solid-state batteries—to commercialization. This article summarizes the team’s work to tackle these challenges over the past three years, which have been reported in several peer-reviewed articles published in various journals.
Unlike today’s rechargeable lithium ion batteries, which contain liquid electrolytes that are often flammable, batteries with solid electrolytes offer the possibility of greater safety, in addition to a whole range of benefits including higher energy density.
In the Nature Nanotechnology review article, the researchers focus on inorganic solid electrolytes such as ceramic oxides or sulfide glasses. Inorganic solid electrolytes are a relatively new class of solid electrolytes for all-solid-state batteries (in contrast to organic solid electrolytes which are more extensively researched.)
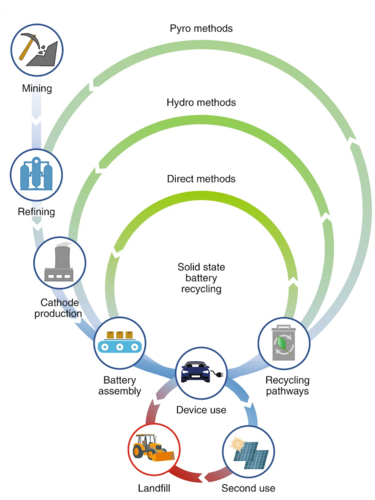
Illustration of various recycling methods with reference to direct battery recycling method proposed in solid state batteries. Image courtesy of Nature Nanotechnology
Roadmap: Inorganic Electrolytes for All-Solid-State Batteries
Illustration of various recycling methods with reference to direct battery recycling method proposed in solid state batteries. Image courtesy of Nature Nanotechnology
The following is an outline of the roadmap that the researchers describe in their review article:
- Creating stable solid electrolyte chemical interfaces
- New tools for in operando diagnosis and characterization
- Scalable and cost-effective manufacturability
- Batteries designed for recyclability
“It’s critical that we step back and think about how to address these challenges simultaneously because they are all interrelated,” said Shirley Meng, a nanoengineering professor at the UC San Diego Jacobs School of Engineering. “If we are going to make good on the promise of all-solid-state batteries, we must find solutions that address all these challenges at the same time.”
As director of the UC San Diego Sustainable Power and Energy Center and director of the UC San Diego Institute for Materials Discovery and Design, Meng is a key member of a cluster of researchers at the forefront of all solid-state battery research and development at UC San Diego.
Creating Stable Solid Electrolyte Chemical Interfaces
Solid-state electrolytes have come a long way since their early days, when the first electrolytes discovered had exhibited conductivity values too low for practical applications. Today’s advanced solid-state electrolytes show conductivities exceeding even those of conventional liquid electrolytes used in today’s batteries (greater than 10 mS cm-1). Ionic conductivity refers to how fast lithium ions can move within the electrolyte.
Unfortunately, most highly conductive solid electrolytes reported are often electrochemically unstable and face problems when applied against electrode materials used in batteries.
“At this point, we should shift our focus away from chasing higher ionic conductivity. Instead, we should focus on stability between solid state electrolytes and electrodes,” said Meng.
If ionic conductivity is analogous to how fast a car can be driven, then interface stability refers to how hard it is to get through rush hour traffic. It doesn’t matter how fast your car can go if you’re stuck in traffic on your way to work.
Researchers at UC San Diego recently addressed this interface stability bottleneck, demonstrating how to stabilize the electrode-electrolyte interface and improve battery performance using solid electrolytes with moderate ionic conductivities but exhibit stable interfaces.
New Tools for In Operando Diagnosis and Characterization
Why do batteries fail? Why does short circuit occur? The process of understanding what goes on inside a battery requires characterization down to the nanoscale, ideally in real time. For all-solid-state batteries, this is immensely challenging.
Battery characterization typically relies on using probes such as X-rays, or electron or optical microscopy. In commercial lithium ion batteries, the liquid electrolytes used are transparent, allowing observation of various phenomena at the respective electrodes. In some cases, this liquid can also be washed away to provide a cleaner surface for higher resolution characterization.
“We have a much easier time observing today’s lithium ion batteries. But in all-solid-state batteries, everything is solid or buried. If you try the same techniques for all-solid-state batteries, it’s like trying to see through a brick wall,” said Darren H. S. Tan, a nanoengineering Ph.D. candidate at the UC San Diego Jacobs School of Engineering.
In addition, solid electrolytes and lithium metal used in solid-state batteries can be sensitive to electron beam damage. This means that standard electron microscopy techniques used to study batteries would damage the materials of interest before they can be observed and characterized.
One way UC San Diego researchers are overcoming these challenges is using cryogenic methods to keep battery materials cool, mitigating their decomposition under the electron microscope probe.
Another tool used to overcome the obstacles of characterizing solid electrolyte interfaces is X-ray tomography. This is similar to what humans undergo during their health checkups. The approach was used in a recent paper reporting on the observation—without opening or disrupting the battery itself—of lithium dendrites buried within the solid electrolyte.
Source:
UC San Diego News Center
Original Publication:
D.H.S. Tan, A. Banerjee, Z. Chen and Y.S. Meng, From nanoscale interface characterization to sustainable energy storage using all-solid-state batteries, Nat. Nanotechnol.15, 170–180 (2020)